Beprin (heparin sodium) solution for injection 5,000 I.U./ml & 1,000 I.U./ml
1 Name of the medicinal product
Beprin 5,000 I.U./ml Solution for injection or concentrate for solution for infusion or Heparin sodium 5,000 I.U./ml Solution for injection or concentrate for solution for infusion
Beprin 1,000 I.U./ml solution for injection or concentrate for solution for infusion or Heparin sodium 1,000 I.U./ml solution for injection or concentrate for solution for infusion
2 Qualitative and quantitative composition
Beprin sodium 5,000 I.U./ml (5,000 I.U. in 1ml, 25,000 I.U. in 5ml)
Beprin sodium 1,000 I.U./ml (5,000 I.U. in 5ml) (10000 I.U. in 10ml)
3 Pharmaceutical form
Solution for injection or concentrate for solution for infusion
A colourless or straw-coloured liquid, free from turbidity and from matter that deposits on standing.
4 Clinical particulars
4.1 Therapeutic indications
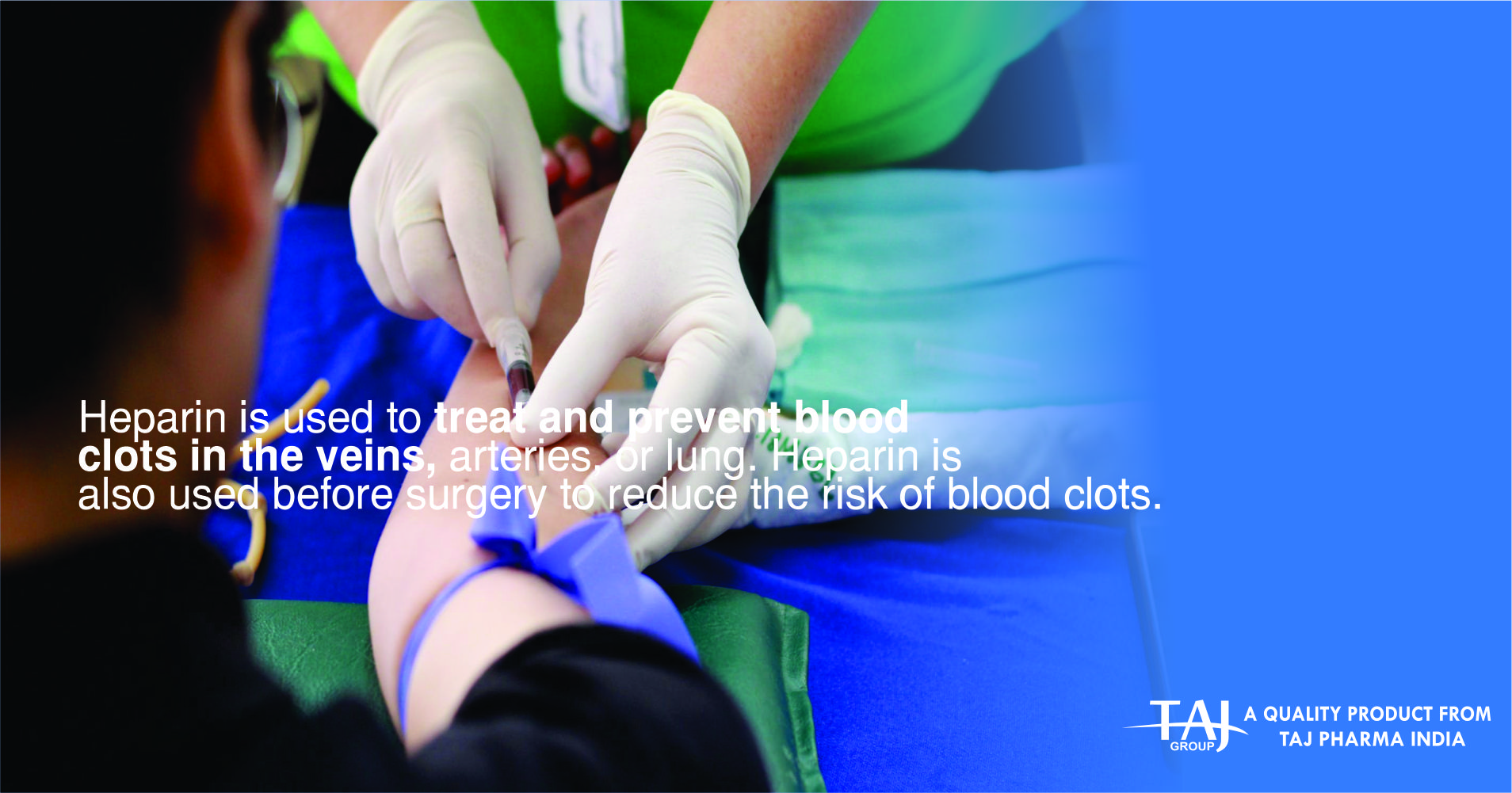
Prophylaxis of deep vein thrombosis and pulmonary embolism
Treatment of deep vein thrombosis, pulmonary embolism, unstable angina pectoris and acute peripheral arterial occlusion.
Prophylaxis of mural thrombosis following myocardial infarction.
In extracorporeal circulation and haemodialysis.
4.2 Posology and method of administration
Route of administration
By continuous intravenous infusion in 5% glucose or 0.9% sodium chloride or by intermittent intravenous injection, or by subcutaneous injection.
As the effects of heparin are short-lived, administration by intravenous infusion or subcutaneous injection is preferable to intermittent intravenous injections.
Recommended dosage
Prophylaxis of deep vein thrombosis and pulmonary embolism
Adults:
| 2 hours preoperatively: | 5,000 units subcutaneously |
| followed by: | 5,000 units subcutaneously every 8-12 hours, for 7-10 days or until the patient is fully ambulant. |
No laboratory monitoring should be necessary during low dose heparin prophylaxis. If monitoring is considered desirable, anti-Xa assays should be used as the activated partial thromboplastin time (APTT) is not significantly prolonged.
| During pregnancy: | 5,000 - 10,000 units every 12 hours, subcutaneously, adjusted according to APTT or anti-Xa assay. |
Elderly:
Dosage reduction and monitoring of APTT may be advisable.
Children:
| Loading dose: | 5,000 units intravenously |
| Maintenance: | 1,000-2,000 units/hour by intravenous infusion,or 5,000-10,000 units 4-hourly by intravenous injection. |
No dosage recommendations.
Treatment of deep vein thrombosis and pulmonary embolism:
Adults:
Elderly:
Dosage reduction may be advisable.
Children and small adults:
Treatment of unstable angina pectoris and acute peripheral arterial occlusion:
Adults:
Elderly:
Dosage reduction may be advisable.
Children and small adults:
| Loading dose: | 50 units/kg intravenously |
| Maintenance: | 15-25 units/kg/hour by intravenous infusion, or 100 units/kg 4-hourly by intravenous injection |
Daily laboratory monitoring (ideally at the same time each day, starting 4-6 hours after initiation of treatment) is essential during full-dose heparin treatment, with adjustment of dosage to maintain an APTT value 1.5-2.5 x midpoint of normal range or control value.
Prophylaxis of mural thrombosis following myocardial infarction
Adults:
12,500 units 12 hourly subcutaneously for at least 10 days.
Elderly:
Dosage reduction may be advisable
In extracorporeal circulation and haemodialysis
Adults:
| Loading dose: | 5,000 units intravenously (10,000 units may be required in severe pulmonary embolism) |
| Maintenance: | 1,000-2,000 units/hour by intravenous infusion, or 10,000-20,000 units 12 hourly subcutaneously, or 5,000-10,000 units 4-hourly by intravenous injection. |
| Loading dose: | 50 units/kg intravenously |
| Maintenance: | 15-25 units/kg/hour by intravenous infusion, or 250 units/kg 12 hourly subcutaneously or 100 units/kg 4-hourly by intravenous injection |
Cardiopulmonary bypass:
Initially 300 units/kg intravenously, adjusted thereafter to maintain the activated clotting time (ACT) in the range 400-500 seconds.
Haemodialysis and haemofiltration:
Initially 1,000-5,000 units,
Maintenance: 1,000-2,000 units/hour, adjusted to maintain clotting time >40 minutes.
Heparin resistance
Patients with altered heparin responsiveness or heparin resistance may require disproportionately higher doses of heparin to achieve the desired effect. Also refer to section 4.4, Special warnings and precautions for use.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the other excipients listed in section 6.1
Patients who consume large amounts of alcohol, who are sensitive to the drug, who are actively bleeding or who have haemophilia or other bleeding disorders, severe liver disease (including oesophageal varices), purpura, severe hypertension, active tuberculosis or increased capillary permeability.
Patients with present or previous thrombocytopenia. The rare occurrence of skin necrosis in patients receiving heparin contra-indicates the further use of heparin either by subcutaneous or intravenous routes because of the risk of thrombocytopenia. Because of the special hazard of post-operative haemorrhage heparin is contra-indicated during surgery of the brain, spinal cord and eye, in procedures at sites where there is a risk of bleeding, in patients that have had recent surgery, and in patients undergoing lumbar puncture or regional anaesthetic block.
The relative risks and benefits of heparin should be carefully assessed in patients with a bleeding tendency or those patients with an actual or potential bleeding site eg. hiatus hernia, peptic ulcer, neoplasm, bacterial endocarditis, retinopathy, bleeding haemorrhoids, suspected intracranial haemorrhage, cerebral thrombosis or threatened abortion.
Menstruation is not a contra-indication.
4.4 Special Warnings and precautions for use
Platelet counts should be measured in patients receiving heparin treatment for longer than 5 days and the treatment should be stopped immediately in those who develop thrombocytopenia.
In patients with advanced renal or hepatic disease, a reduction in dosage may be necessary. The risk of bleeding is increased with severe renal impairment and in the elderly (particularly elderly women).
Although heparin hypersensitivity is rare, it is advisable to give a trial dose of 1,000 I.U. in patients with a history of allergy. Caution should be exercised in patients with known hypersensitivity to low molecular weight heparins.
In most patients, the recommended low-dose regimen produces no alteration in clotting time. However, patients show an individual response to heparin, and it is therefore essential that the effect of therapy on coagulation time should be monitored in patients undergoing major surgery.
Caution is recommended in spinal or epidural anaesthesia (risk of spinal haematoma).
Heparin can suppress adrenal secretion of aldosterone leading to hyperkalemia, particularly in patients such as those with diabetes mellitus, chronic renal failure, pre-existing metabolic acidosis, a raised plasma potassium, or taking potassium sparing drugs. The risk of hyperkalemia appears to increase with duration of therapy but is usually reversible. Plasma potassium should be measured in patients at risk before starting heparin therapy and in all patients treated for more than 7 days.
Heparin resistance
There is considerable variation in individual anticoagulant responses to heparin.
Heparin resistance, defined as an inadequate response to heparin at a standard dose for achieving a therapeutic goal occurs in approximately 5 to 30% of patients.
Factors predisposing to the development of heparin resistance, include:
• Antithrombin III activity less than 60% of normal (antithrombin III-dependent heparin resistance):
Reduced antithrombin III activity may be hereditary or more commonly, acquired (secondary to preoperative heparin therapy in the main, chronic liver disease, nephrotic syndrome, cardiopulmonary bypass, low grade disseminated intravascular coagulation or drug induced, e.g. by aprotinin, oestrogen or possibly nitroglycerin)
• Patients with normal or supranormal antithrombin III levels (antithrombin III-independent heparin resistance)
• Thromboembolic disorders
• Increased heparin clearance
• Elevated levels of heparin binding proteins, factor VIII, von Willebrand factor, fibrinogen, platelet factor 4 or histidine-rich glycoprotein
• Active infection (sepsis or endocarditis)
• Preoperative intra-aortic balloon counterpulsation
• Thrombocytopenia
• Thrombocytosis
• Advanced age
• Plasma albumin concentration ≤ 35g/dl
• Relative hypovolaemia
Heparin resistance is also often encountered in acutely ill patients, in patients with malignancy and during pregnancy or the post-partum period.
4.5 Interaction with other medicinal products and other forms of interaction
Analgesics: Drugs that interfere with platelet aggregation eg. aspirin and other NSAIDs should be used with care. Increased risk of haemorrhage with ketorolac (avoid concomitant use even with low-dose heparin).
Anticoagulants, platelet inhibitors, etc: Increased risk of bleeding with oral anticoagulants, epoprostenol, clopidogrel, ticlopidine, streptokinase, dipyridamole, dextran solutions or any other drug which may interfere with coagulation.
Cephalosporins: Some cephalosporins, e.g. cefaclor, cefixime and ceftriaxone, can affect the coagulation process and may therefore increase the risk of haemorrhage when used concurrently with heparin.
ACE inhibitors: Hyperkalaemia may occur with concomitant use.
Nitrates: Reduced activity of heparin has been reported with simultaneous intravenous glyceryl trinitrate infusion.
Probenecid: May increase the anticoagulant effects of heparin.
Tobacco smoke: Nicotine may partially counteract the anticoagulant effect of heparin. Increased heparin dosage may be required in smokers.
Interference with diagnostic tests may be associated with pseudo-hypocalcaemia (in haemodialysis patients), artefactual increases in total thyroxine and triiodothyronine, simulated metabolic acidosis and inhibition of the chromogenic lysate assay for endotoxin. Heparin may interfere with the determination of aminoglycosides by immunoassays.
4.6 Fertility, Pregnancy and lactation
Heparin is not contraindicated in pregnancy. Heparin does not cross the placenta or appear in breast milk. The decision to use heparin in pregnancy should be taken after evaluation of the risk/benefit in any particular circumstances.
Reduced bone density has been reported with prolonged heparin treatment during pregnancy.
Haemorrhage may be a problem during pregnancy or after delivery.
4.7 Effects on ability to drive and use machines
None stated.
4.8 Undesirable Effects
Haemorrhage (see also Special Warnings and Precautions and Overdosage Information).
Adrenal insufficiency secondary to adrenal haemorrhage has been associated with heparin (rarely).
Thrombocytopenia has been observed occasionally (see also Special Precautions and Warnings). Two types of heparin-induced thrombocytopenia have been defined. Type I is frequent, mild (usually >50 x 109/L) and transient, occurring within 1-5 days of heparin administration. Type II is less frequent but often associated with severe thrombocytopenia (usually <50 x 109/L). It is immune-mediated and occurs after a week or more (earlier in patients previously exposed to heparin). It is associated with the production of a platelet-aggregating antibody and thromboembolic complications which may precede the onset of thrombocytopenia. Heparin should be discontinued immediately.
There is some evidence that prolonged dosing with heparin (ie. over many months) may cause alopecia and osteoporosis. Significant bone demineralisation has been reported in women taking more than 10,000 I.U. per day of heparin for at least 6 months.
Heparin products can cause hypoaldosteronism which may result in an increase in plasma potassium. Rarely, clinically significant hyperkalemia may occur particularly in patients with chronic renal failure and diabetes mellitus (see Warnings and Precautions).
Hypersensitivity reactions to heparin are rare. They include urticaria, conjunctivitis, rhinitis, asthma, cyanosis, tachypnoea, feeling of oppression, fever, chills, angioneurotic oedema and anaphylactic shock.
Local irritation and skin necrosis may occur but are rare. Erythematous nodules, or infiltrated and sometimes eczema-like plaques, at the site of subcutaneous injections are common, occurring 3-21 days after starting heparin treatment.
Priapism has been reported. Increased serum transaminase values may occur but usually resolve on discontinuation of heparin. Heparin administration is associated with release of lipoprotein lipase into the plasma; rebound hyperlipidaemia may follow heparin withdrawal.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store
4.9 Overdose
A potential hazard of heparin therapy is haemorrhage, but this is usually due to overdosage and the risk is minimised by strict laboratory control. Slight haemorrhage can usually be treated by withdrawing the drug. If bleeding is more severe, clotting time and platelet count should be determined. Prolonged clotting time will indicate the presence of an excessive anticoagulant effect requiring neutralisation by intravenous protamine sulfate, at a dosage of 1 mg for every 100 I.U. of heparin to be neutralised. The bolus dose of protamine sulfate should be given slowly over about 10 minutes and not exceed 50 mg. If more than 15 minutes have elapsed since the injection of heparin, lower doses of protamine will be necessary.
5.1 Pharmacodynamic properties
Heparin is an anticoagulant and acts by inhibiting thrombin and by potentiating the naturally occurring inhibitors of activated Factor X (Xa).
5.2 Pharmacokinetic properties
As heparin is not absorbed from the gastrointestinal tract and sublingual sites it is administered by injection. After injection heparin extensively binds to plasma proteins.
Heparin is metabolised in the liver and the inactive metabolic products are excreted in the urine.
The half life of heparin is dependent on the dose.
5.3 Preclinical safety data
There are no pre-clinical data of relevance to the prescriber which are additional to those already included in other sections.
6 Pharmaceutical particulars
6.1 List of excipients
Benzyl alcohol
Methyl parahydroxybenzoate (E218)
Water for injections
Sodium hydroxide solution 3M
Hydrochloric acid 3M
6.2 Incompatibilities
Heparin is incompatible with many injectable preparations e.g. some antibiotics, opioid analgesics and antihistamines.
The following drugs are incompatible with heparin;
Alteplase, amikacin sulfate, amiodarone hydrochloride, ampicillin sodium, aprotinin, benzylpenicillin potassium or sodium, cefalotin sodium, chlorpromazine hydrochloride, ciprofloxacin lactate, cisatracurium besilate, cytarabine, dacarbazine, daunorubicin hydrochloride, diazepam, doxorubicin hydrochloride, droperidol, erythromycin lactobionate, gentamicin sulfate, haloperidol lactate, hyaluronidase, hydrocortisone sodium succinate, kanamycin sulfate, labetolol hydrochloride, meticillin sodium, methotrimeprazine, netilmicin sulfate, nicardipine hydrochloride, oxytetracycline hydrochloride, pethidine hydrochloride, polymyxin B sulfate, promethazine hydrochloride, streptomycin sulfate, tobramycin sulfate, triflupromazine hydrochloride, vancomycin hydrochloride and vinblastine sulfate.
Dobutamine hydrochloride and heparin should not be mixed or infused through the same intravenous line, as this causes precipitation.
Heparin and reteplase are incompatible when combined in solution.
If reteplase and heparin are to be given through the same line this, together with any Y-lines, must be thoroughly flushed with a 0.9% saline or a 5% glucose solution prior to and following the reteplase injection.
6.3 Shelf life
Unopened – 3 years
From a microbiological point of view, unless the method of opening precludes the risk of microbial contamination, the product should be used immediately.
If not used immediately, in-use storage times and conditions are the responsibility of the user.
6.4 Special precautions for storage
Do not store above 25°C.
Store in original container.
6.5 Nature and contents of container
Neutral glass ampoules (Type I Ph Eur) of 1ml or 2ml capacity and 5ml capacity containing 1ml and 5ml of solution respectively. Cartons contain 10 ampoules.
6.6 Special precautions for disposal and other handling
Not applicable
7 Manufacturer:
Manufactured in India by:
TAJ PHARMACEUTICALS LTD,
220, Mahagujarat Ind. Estate, Moraiya,
Tal. Sanand , Dist. Ahmedabad,
Gujarat, INDIA.
Click here for Download pdf of patient informationClick here for Download pdf of prescribing information
Our Partners
A dream for new world Anchored in India and committed to its traditional values of leadership with trust, the Taj Pharma Group is spreading its footprint globally through excellence and innovation.Taj Pahrma India.














Important Safety Information
Allergy warning
Beprin can cause a severe allergic reaction. Symptoms can include: skin tissue death at the injection site, chills, fever, rash, hives, itching, burning, shortness of breath, swelling of your face, lips, throat, or tongue If you develop these symptoms, go to the nearest emergency room.
Don’t take this drug again if you’ve ever had an allergic reaction to it. Beprin is derived from animal tissue. It should be used with caution in people with a history of allergy to this drug or to pig proteins. Taking beprin could be fatal (cause death).
Warnings for people with certain health conditions
For people with pig protein sensitivity: Do not take this drug. This drug is made from pork tissue and can cause a life-threatening allergic reaction in people who are sensitive or allergic to other pig proteins.
For people with uncontrolled high blood pressure: You are at an increased risk of bleeding from this drug. Talk to your doctor before using beprin.
For people with bleeding or clotting problems: If you have abnormal bleeding or a condition that puts you at an increased risk of bleeding, using beprin could increase your risk even more. Use Beprin with caution.
For people with a history of stomach ulcers or bleeding: If you have active stomach ulcers, you should not use beprin. It could make your ulcers worse and cause dangerous bleeding. If you have a history of stomach ulcers but don’t have active ulcers, using beprin puts you at an increased risk of bleeding. You should talk to your doctor before using beprin.
For people with kidney disease: If you have severe kidney disease or a history of kidney disease, taking beprin can increase your risk of bleeding. Talk to your doctor before using beprin.
For people with liver disease: If you have severe liver disease or a history of liver disease, taking beprin can increase your risk of bleeding. Talk to your doctor before using beprin.
For people with asthma or sulfite sensitivity: People with asthma are likely to be sensitive to sulfites. Sulfites can cause a life-threatening allergic reaction in some people. Some forms of this drug contain sulfites. Talk to your doctor about using a sulfite-free version of beprin.
Warnings for other groups
For pregnant women: Research in animals has shown negative effects to the fetus when the mother uses beprin. However, there haven’t been enough studies done in humans to be certain how the drug might affect the fetus. Talk to your doctor if you’re pregnant or plan to become pregnant. This drug should only be used if the potential benefit justifies the potential risk. Ask your doctor if using the preservative-free version of beprin would be better for you than the version that contains benzyl alcohol. If you become pregnant while taking this drug, call your doctor right away.
For women who are breastfeeding: Beprin is unlikely to pass into breast milk and be absorbed by an infant who is breastfed. Talk to your doctor about the best way to feed your child while you’re taking beprin. Some forms of beprin contain a preservative called benzyl alcohol. This ingredient can slow down the central nervous system in some infants. It has also caused trouble breathing and changes in the blood chemistry in some infants. These effects can be deadly. If you breastfeed your child, talk to your doctor about preservative-free beprin.
For seniors: If you are older than 60 years, you may be at a higher risk of bleeding. Beprin also increases your risk of bleeding, so your doctor may start you on a reduced dosage.
For children: This medication has not been studied in children. Dosage recommendations are based on clinical experience. Newborns and infants should receive preservative-free beprin. The preservative benzyl alcohol has been linked to serious side effects and even death in newborns and infants.